Fluorescent Photosensitizer Vectors
Genetically-encoded Photosensitizers
Light-induced cell killing
- Light-induced production of reactive oxygen species
- Direct expression in cells, easy targeting to various subcellular compartments
- No exogenous chemical compounds required for chromophore maturation
- Not toxic before activation by light irradiation
- Recommended for selective light-induced protein inactivation and cell killing
Genetically-encoded photosensitizers KillerRed and KillerOrange represent fluorescent proteins of the GFP family that generate reactive oxygen species (ROS) upon light irradiation with certain wavelengths: 540-590 nm for KillerRed and 450-540 nm for KillerOrange. The photosensitizers are not toxic before activation by light irradiation.
KillerRed and KillerOrange can be used for selective light-induced killing of the cells. The level of cytotoxic effect and type of the cell death are dependent on the intracellular localization of the photosensitizers, their concentration, light intensity and time of irraditaion. The photosensitizers can be also applied for chromophore-assisted light inactivation (CALI) technology.
Due to the substantially different excitation spectra, the KillerOrange-KillerRed pair can potentially be used to independently ablate two cell populations. This pair also promises the orthogonal optical control of the propagation of signaling cascades either by chromophore-assisted light inactivation of the participating proteins or by triggering cascades with hydrogen peroxide produced by KillerRed and likely by KillerOrange. KillerOrange-KillerRed tandem fusions or combination of various photosensitizers in one cassette may enhance phototoxicity under white light irradiation and may be useful as a research tool in biology.
KillerRed
Red fluorescent protein KillerRed is the first genetically-encoded photosensitizer [Bulina et al., 2006a]. Unlike chemical analogs, KillerRed can be directly expressed by target cells, both individually and in fusion with a target protein. It shows no cell toxic effects before light activation. Upon green or orange light irradiation, KillerRed generates ROS that damage the neighboring molecules.
Bulina ME, Chudakov DM, Britanova OV, Yanushevich YG, Staroverov DB, Chepurnykh TV, Merzlyak EM, Shkrob MA, Lukyanov S, Lukyanov KA. A genetically encoded photosensitizer. Nat Biotechnol. 2006a; 24 (1):95-9. / pmid: 16369538
Genetically-encoded Photoinducible Cell Cycle Inhibitor ArrestRed
- Reversible inhibition of cell cycle progression
- Activation by green light irradiation
- Direct expression and easy visualization in cell nuclei
- No exogenous chemical compounds required
- Recommended for transient blockage of cell division in vitro and in vivo
ArrestRed (the scientific name is H2B-tKR) is a novel optogenetic tool, which can be used to study the roles of specific cell populations in development, regeneration, and carcinogenesis. This is a chimeric protein composed of a tandem version of genetically-encoded photosensitizer KillerRed fused to histone H2B protein [Serebrovskaya et al., 2011]. Expression of ArrestRed in mammalian cells results in correct chromatin labeling and does not interfere with cellular division. The illumination of the ArrestRed expressing cells by intense green light leads to blockage of cell proliferation. The effect of ArrestRed activation lasts for about 24 hours, after that approximately 90% of ArrestRed expressing cells resume division. Repeated light illuminations allow to maintain cells in the non-dividing state for longer periods.
The inhibitory effect of ArrestRed on cell cycle progression is attributed to generation of reactive oxygen species (ROS) upon ArrestRed activation [Bulina et al., 2006]. Light-induced generation of ROS leads to massive damage of genomic DNA and activation of repair machinery. In turn, it causes cell cycle checkpoints activation and cell cycle arrest. After successful DNA reparation interphase cells restore normal proliferation.
Bulina ME, Chudakov DM, Britanova OV, Yanushevich YG, Staroverov DB, Chepurnykh TV, Merzlyak EM, Shkrob MA, Lukyanov S, Lukyanov KA. A genetically encoded photosensitizer. Nat Biotechnol. 2006; 24 (1):95-9. / pmid: 16369538
Serebrovskaya EO, Gorodnicheva TV, Ermakova GV, Solovieva EA, Sharonov GV, Zagaynova EV, Chudakov DM, Lukyanov S, Zaraisky AG, Lukyanov KA. Light-induced blockage of cell division with a chromatin-targeted phototoxic fluorescent protein. Biochem J. 2011; 435 (1):65-71. doi: 10.1042/BJ20101217 / pmid: 21214518
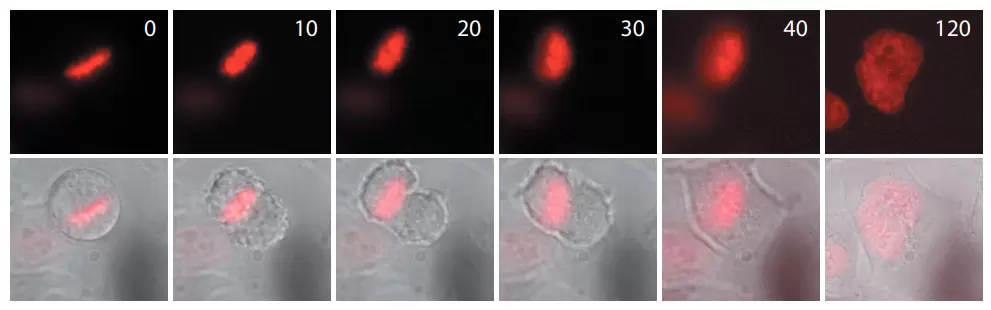
Time-lapse images of a representative HeLa cell expressing ArrestRed.
Activation of ArrestRed in mitotic cells results in nondisjunction of chromosomes, and the cells are unable to complete division normally. In the end the cells return to interphase morphology with a decondensed tetraploid chromatin. Activation of ArrestRed was performed during metaphase (numbers indicate time in minutes from induction; red fluorescence and overlay of red fluorescence and transmitted light are shown).